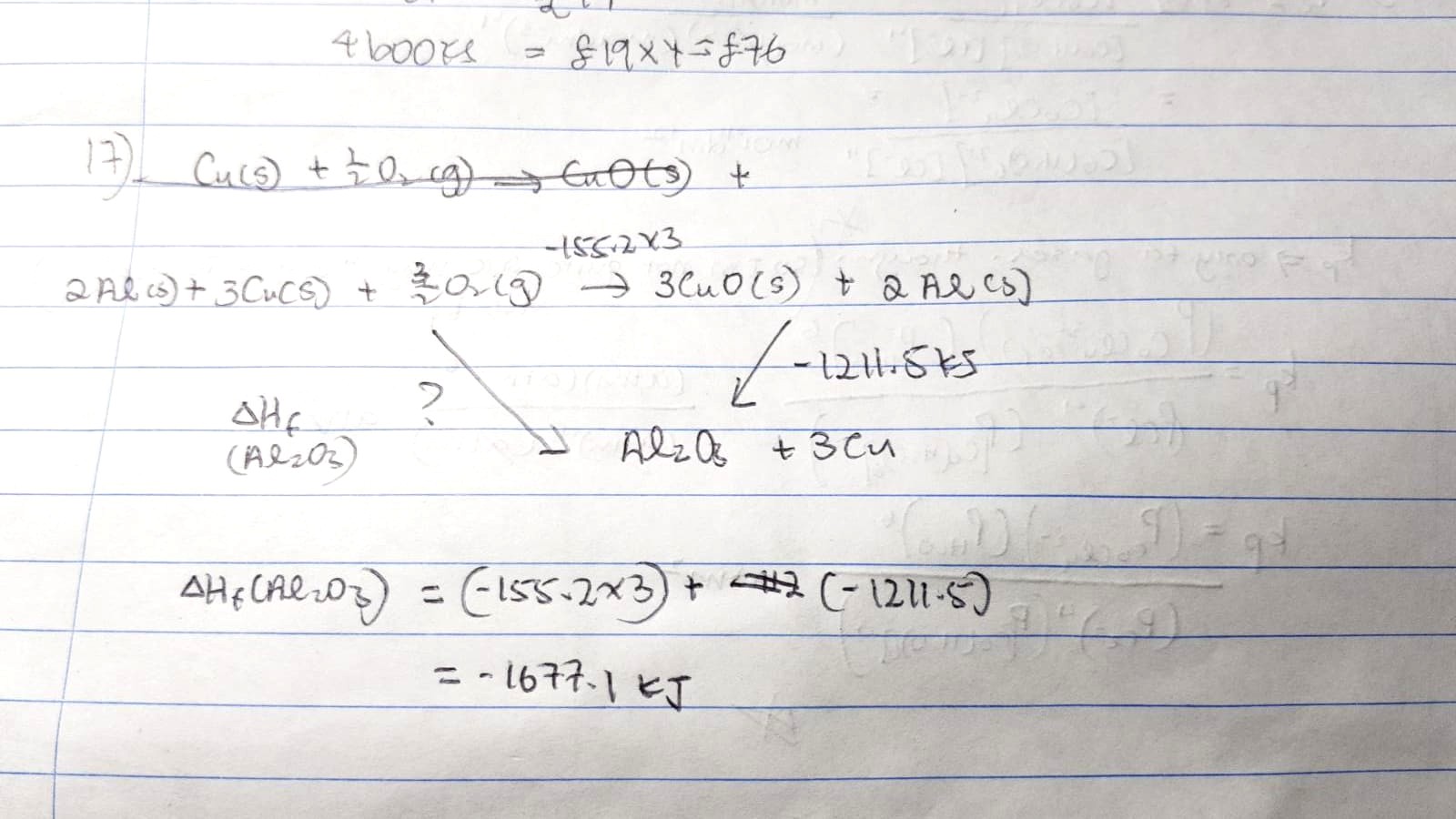Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
the Answer is D. Since the question gave you 2 equations, you have to manipulate both equations such that it can form an energy cycle. Then using Hess's Law, find the enthalpy change of formation of Aluminium oxide. Hope this helps !
Date Posted:
5 years ago