Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
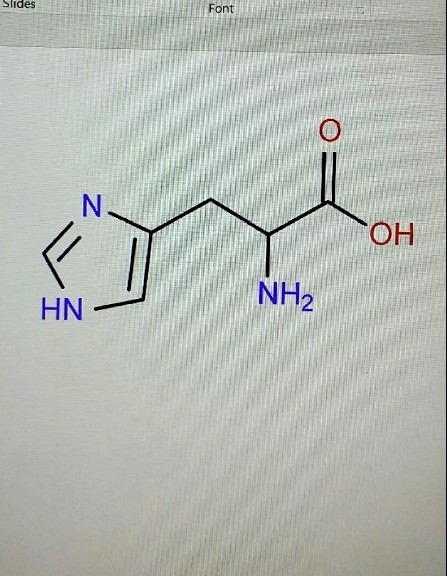
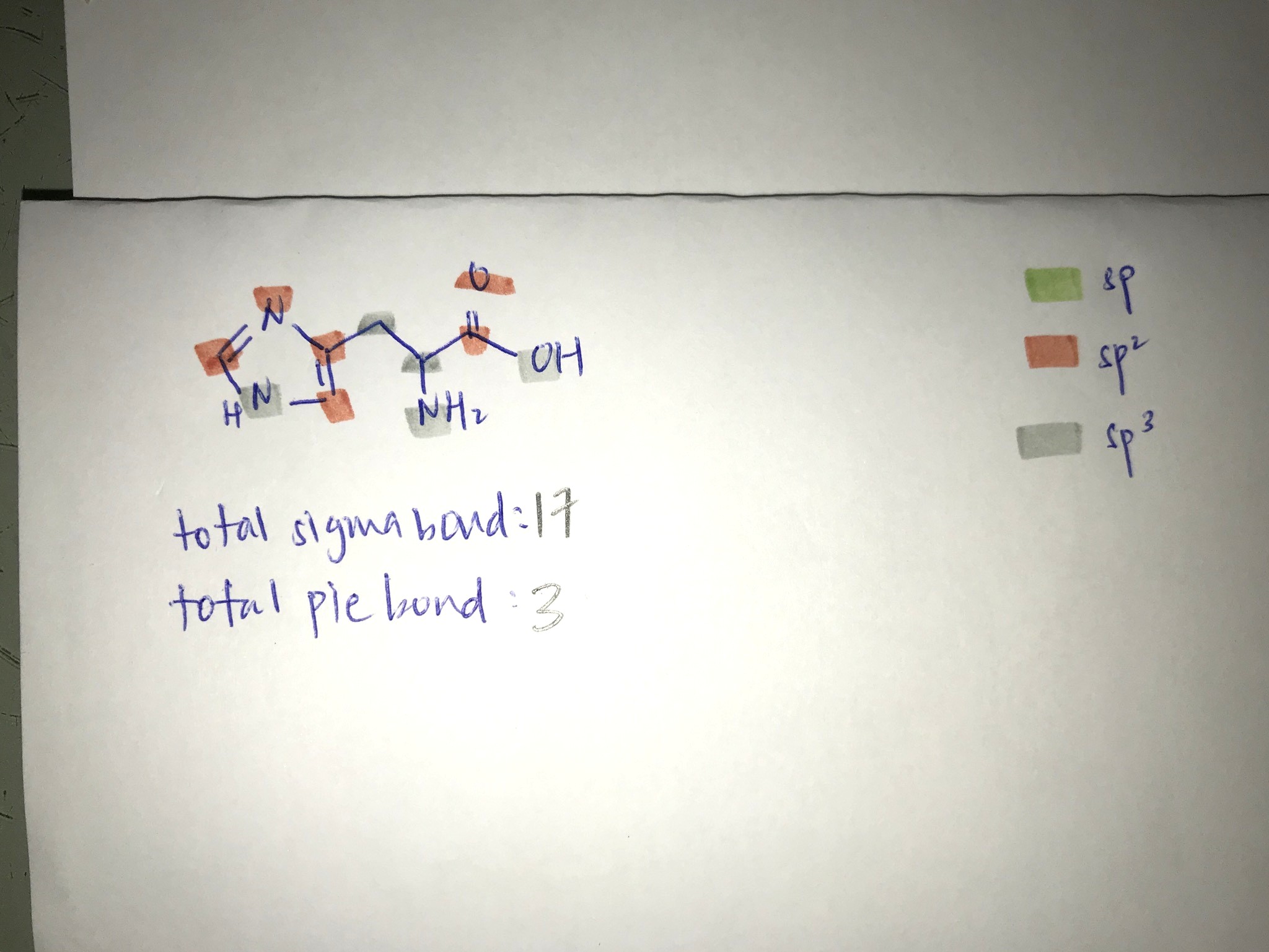
Usually, we are more interested in the hybridisation state of carbon atoms but it is also possible to deduce hybridisation state of oxygen and nitrogen.
Identify how many lone pairs and bond pairs each carbon/oxygen/nitrogen atoms are there, then we can deduce what hybridisation state it is.
Pie bond is usually the double bond that you see.
I hope this answer is clear enough for you!
Identify how many lone pairs and bond pairs each carbon/oxygen/nitrogen atoms are there, then we can deduce what hybridisation state it is.
Pie bond is usually the double bond that you see.
I hope this answer is clear enough for you!
Date Posted:
5 years ago
The N of the NH of the imidazole ring should be sp2 instead of sp3. This is what gives the imidazole ring it's aromaticity. (Lone pair is in the p orbital). Being sp3 would destroy the aromaticity and destabilise the ring.
The O of the OH of the carboxylic acid group should be sp2 so that it is conjugated/in resonance with the carbonyl group.
The O of the OH of the carboxylic acid group should be sp2 so that it is conjugated/in resonance with the carbonyl group.