Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 1 | H1 Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

Need help with question 6!
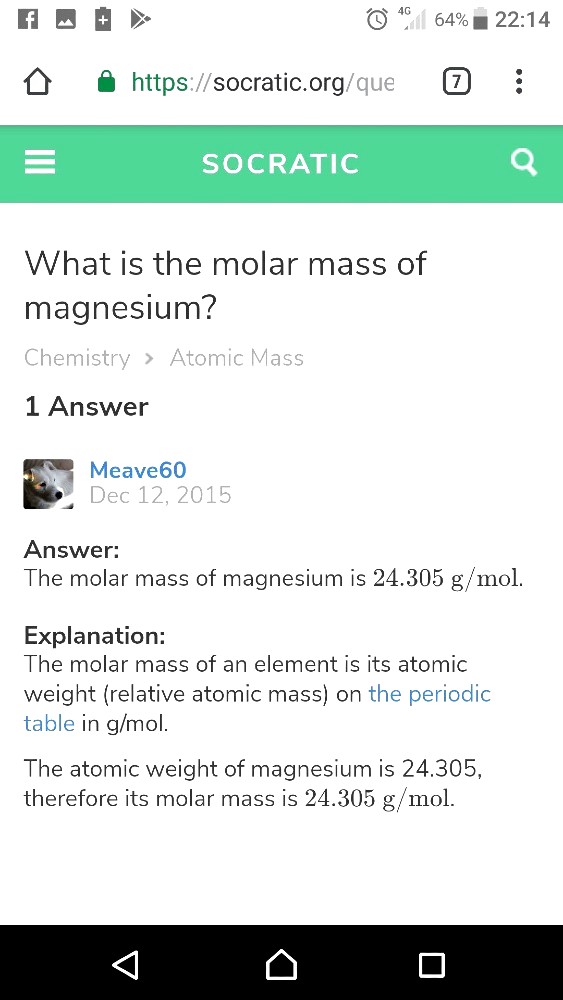
9.681 x 10^(-3) M Mg2+
= 9.681 x 10^(-3) mol/l
= 9.681 x 10^(-4) mol/100ml
(Recall 1l = 1000ml. So to get /100ml, ÷10 which also means x10^(-1) )
= 9.681 x 10^(-4)mol x 24.305g/mol /100ml
= 0.0235296705 g/100ml
= 0.0235 % m/v (3s.f)
The volume of sample is not needed as the concentration is what we are finding. Volume is required if you are finding the total mass or number of mol of Mg2+
See 1 Answer