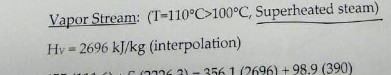Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 2 | H2 Maths
5 Answers Below
Anyone can contribute an answer, even non-tutors.

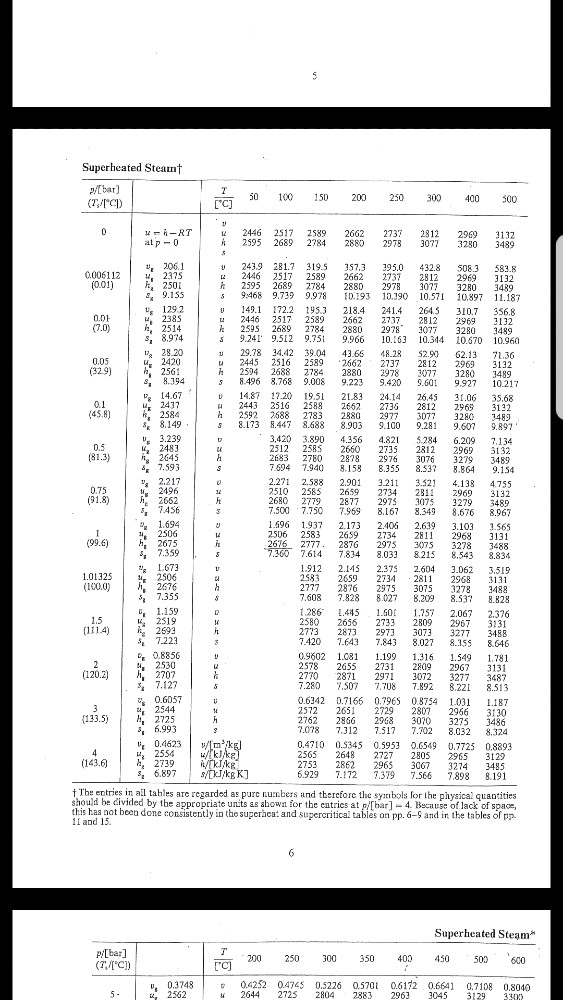
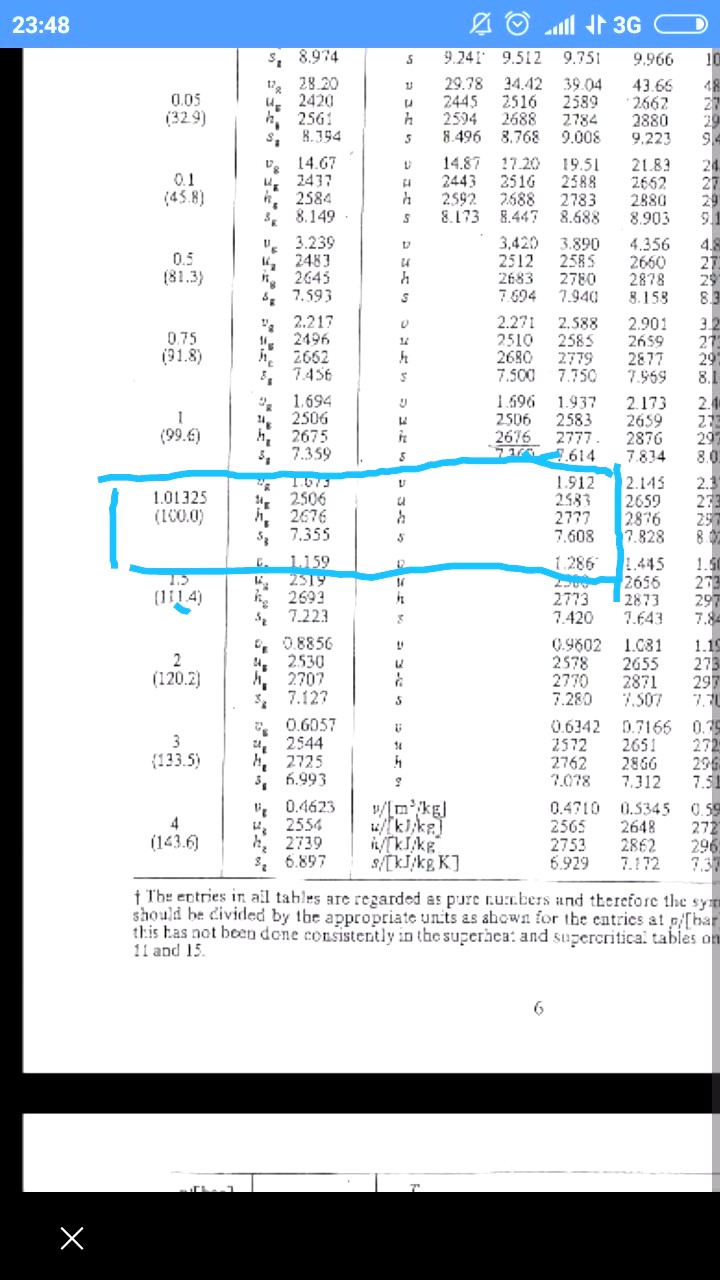
I got 2690 when i interpolate
See 5 Answers

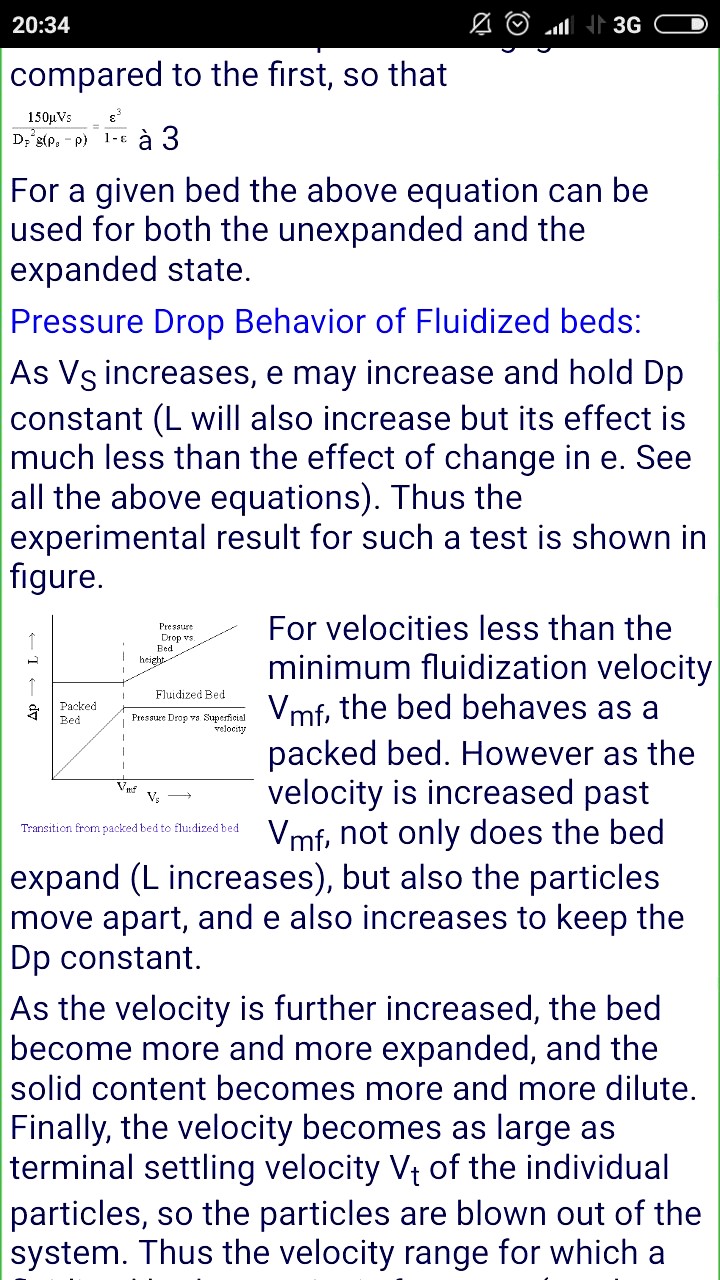
if mass of the catalyst particles increases , what will be the effect on the type of fluidization ?
if mass of the catalyst particles decreases , what will be the effect on the type of fluidization ?
The weight of the particles = buoyancy of the air flow, is the condition of fluidization.
What do you mean by fluidization type

But i thought vmf increase good for the fluidization?
Sorry and help me please thank you
organochlorine pesticides in herb
product by gas chromatography‐ mass
spectrometry
Can tell me what i have to research on? My senior told me to research on
1)Performance characteristic on method validation
2)what is method validation and development
Why its important
3)development of methods thar are used to detect organochlorine pesticides from the old days to now
Is thar the only thing i have to research? No right ?
Pesticide residue analysis in food samples have evolved steadily in the last 40 years. In 1963 , the first method to develop for the detection of organochlorine was produced. Acetonitrile was used as the extraction solvent and its partitioning with petroleum ether was used for sample clean up .
As the requirements for method increases throughout the years ,the analytical range also expanded to include organophosphorus and organonitrogen pesticides. Two chemist , Becker and Luke realized that the non-polar solvents (acetonitrile and petroleum ether) used in previous method causes a partial loss of the more polar pesticides. They decided to switched the initial extraction solvent from acetonitrile to acetone.
This result in the analytical range to be expanded . However, non-polar solvents such as methylene chloride or petroleum ether were still used for partitioning. Salts such as NaCl, were added during the partitioning steps to enhance the recoveries of polar analytes.
These methods continue to be used, as detection limit becomes lower. Recent development and improvement in analytical instrumentation make this possible.
In 1993, the original ‘Luke’ Method was modified and is now referred to as Luke II Method . This method introduced solid-phase extraction (SPE) clean up set ups in both pre- and post-partitioning step. Other combination of appropriate detection technique include; GC/MS(Gas Chromatography/Mass Spectrometry) , GC/ECD(Gas Chromatography/Electron Capture Detector) , GC/NPD(Nitrogen-Phosphorous detector). These detection techniques proved that methods could be reduced to approximately 10ppb (parts per billion) for an incurred pesticide sample. Water was used as an integral part of the initial extraction to facilitate SPE loading.
However, the sample preparation procedures for this method were complicated and tedious, taking as long as 1.5days. It requires a large amount of solvent, lengthy evaporation steps and extensive use of glassware.
In 1990, many new extraction techniques were produced. They are MAE (microwave-assisted extraction, ASE (accelerated solvent extraction) and SFE (supercritical fluid extraction). Although they have individual advantages, these techniques were not widely accepted due to various practical limitations . For instance, ASE require significant numbers of preparative steps and a higher maintenance cost.
To enhance the efficiency of traditional methods, a new sample preparation approach known as QuEChERS was introduced by Anastassiades. The anagram for ‘QuEChERS’ is Quick-Easy-Cheap-Effective-Rugged-Safe.
This approach requires a single extraction in acetonitrile and require a very small sample size (10-15g). Large excess of salts and buffers are added. Excess salts are used to aid in the extraction of polar and non-polar pesticides whereas buffers are used to improve analyte stability and extract quality. This initial step simultaneously extracts the pesticides from the sample , preparing it for dispersive solid-phase extraction (d-SPE) step. The d-SPE step helps to remove residual water and further removal of matrix interferences from the sample.
All in all, QuEChERS are developed due to the 40 years of evolution of all type of methods and techniques.The QuEChERS method has become the most popular method to detect pesticides such as organochlorine. Due to its ability to allow more samples to be screened for a large quantity of compounds in a shorter period of time compared to the other previous methods, this technique is rapidly gaining acceptance across the globe .
2. What is method development and validation ? Why is it important ?
Analytical method development and validation are the continuous and inter-dependent task associated with the research and development, quality control and quality assurance departments. Analytical procedures play a critical role in equivalence and risk assessment management. It helps in formation of product-specific acceptance criteria and stability of results.Validation shows that the analytical procedure is suitable for its intented purpose.
Designing of experiment is a powerful tool for the method characterization and validation. Analytical professionals should be capable of using it to characterize and optimize the analytical method. Effective analytical method development and its validation can deliver significant improvements in precision and a reduction in bias errors. It can further help to avoid costly and time consuming exercises.
Analytical method development uses advanced technologies to determine the compositions of a formulation by analytical techniques. It is the process of proving that an analytical method is acceptable for use in laboratory to measure the concentration of subsequent samples. Analytical instruments play a major role in this process to achieve high quality and reliable analytical data. Thus, everyone in the analytical laboratory should be aware about the quality assurance of the equipments.
Analytical method could be spectral, chromatographic, electrochemical, hyphenated or miscellaneous. It should be used within GMP(Good Manufacturing Practice) and GLP (Good Laboratory Practice) environments and must be developed using the protocols and acceptance criteria set out in the ICH guidelines.
An analytical procedure is developed to test a defined characteristic of the substance against the acceptance criteria for that particular characteristic. In the development of a new analytical procedure, the choice of analytical instrumentation and methodology should be based on the intended purpose and scope of the analytical method.
The important parameters that may be assessed during method development are :specificity, linearity, LOD(limits of detection) and LOQ (quantitation limits), range, accuracy and precision During early stages of method development, the robustness of methods should be evaluated because this characteristic helps to determine which method will be approved . Analytical procedures development are primarily based on a combination of mechanistic understanding of the basic methodology and prior experiences. Experimental data from early procedures can be used to guide further development.
The ability to present accurate, reliable and consistent data is the motive of the analytical chemist. Method development procedures are complex, extended and expensive endeavours. An analytical method details the steps and techniques necessary to perform an analysis. This may include: preparation of samples, standards and reagents; use of apparatus; generation of the calibration curve, use of the formulae for the calculation
The need of validation of the analytical method development emerged due to international competition, maintaining the standard of products in high commercial & market value and ethical reasons. Various International Regulatory Agencies have set the standard and fixed the protocol to match the reference for granting approval, authentication and registration.
Data quality is assured by the combination of four components: analytical instrument qualification (AIQ); analytical method validation; system suitability tests and quality control checks. Validation of an analytical method is intended to demonstrate that it is suitable for its intended use.
The type of method and analytical technique used will control the nature and extent of the validation studies required. The most common methods for validation are identification, assay and impurities determination.
The validation of an analytic method demonstrates the scientific soundness of the measurement or characterization. It is required for various extents throughout the regulatory submission process. The validation practice shows that an analytic method measures the correct substance, in the correct amount and in the appropriate range for the samples. It enables the analyst to understand the behavior of the method and to establish the performance limits of the method.
In order to carry out method validation, the laboratory should follow a written standard operating procedure (SOP) that describes the process to conduct it. The laboratory should use qualified and calibrated instrumentation. There should be a well developed ,documented test method and an approved protocol prior to validation. The protocol is a methodical plan that describes which method performance parameters should be tested, how the parameters will be evaluated with its acceptance criteria. Below are the parameters that may be assessed during method validation.
In conclusion , method development and validation are important as they are critical elements of pharmaceutical development hence it is important to develop efficient and accurately validated analytical methods to provide safe and effective drugs for human consumption. Hence, rapid and accurate quantification of the substrate and drug product is important in the process development.
1.Performance characteristic on method validation
Method validation (MV) is sometimes referred to as the process of providing documented evidence about what a method is intended to do. Laboratories in the pharmaceutical and other regulated industries must perform MV to comply with regulations.
In MV, several performance characteristics may be investigated, depending on the type of method and its intended use. These are summarized below.
1)Accuracy is the closeness of agreement between the values found. The value accepted as a conventional true value or the accepted reference value. Several methods of determining accuracy are available:
1) It can be screened by the use of an analytical procedure to an analyte of known purity,2) by comparison of the results of the proposed analytical procedure with those of a second accepted procedure, 3)the accuracy of which is stated and defined. It can also be deduced once precision, linearity and specificity have been established.
2)Precision of an analytical procedure expresses the closeness of agreement between a series of measurements obtained from multiple sampling of the same homogeneous sample under the prescribed conditions. It can be further branched into a)repeatability, b)intermediate precision and c)reproducibility. The standard deviation, relative standard deviation like coefficient of variation and confidence interval should be reported for each type of precision investigated.
a) Repeatability should be assessed using a minimum of 9 determinations covering the specified range for the procedure by 3 replicates or 6 determinations at 100% of the test concentration.
b)Immediate precision depends on the circumstances under which the procedure is intended to be used. For example, the specific day, analyst performing and equipment used are the random events that cast effect on the precision of the analytical procedure.
c)Reproducibility is assessed by means of an inter-laboratory trial. Reproducibility should be considered in case of the standardization of an analytical procedure.
3) Specificity is the ability to assess the analyte for the presence of various components that may be present. It can be established by a number of approaches, depending on the intended purpose of the method. The ability of the method to assess the analyte of interest in a drug product is determined by a check for interference by placebo. Lack of specificity of an individual analytical procedure may be compensated by other supporting analytical procedures.
4) The quantitation limit of an individual analytical procedure can be understood as the smallest amount of analyte in a sample that can be determined quantitatively with appropriate precision and accuracy. Used primarily to determine impurities and/or degrades in products this is a parameter of quantitative assays for estimating the low levels of compounds in sample matrices
4) The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantitated as an exact value..
6. The linearity of an analytical procedure is its ability to obtain test results that are directly proportional to the concentration of analyte in the sample.
7. The range of an analytical procedure is the interval between the upper and lower concentration of analyte in the sample for which it has been demonstrated that the analytical procedure has a suitable level of precision, accuracy and linearity.
8. Robustness is typically assessed by the effect of small changes in chromatographic methods on system suitability parameters such as peak retention, resolution and efficiency.