Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | Biology
One Answer Below
Anyone can contribute an answer, even non-tutors.

(Chemistry) What is the most important thing I should take note of when doing mole questions? Are there any common mistakes made by students? For some reason moles is my worst topic; I just don’t get it. Some advice is much appreciated!!!:”)))
See 1 Answer
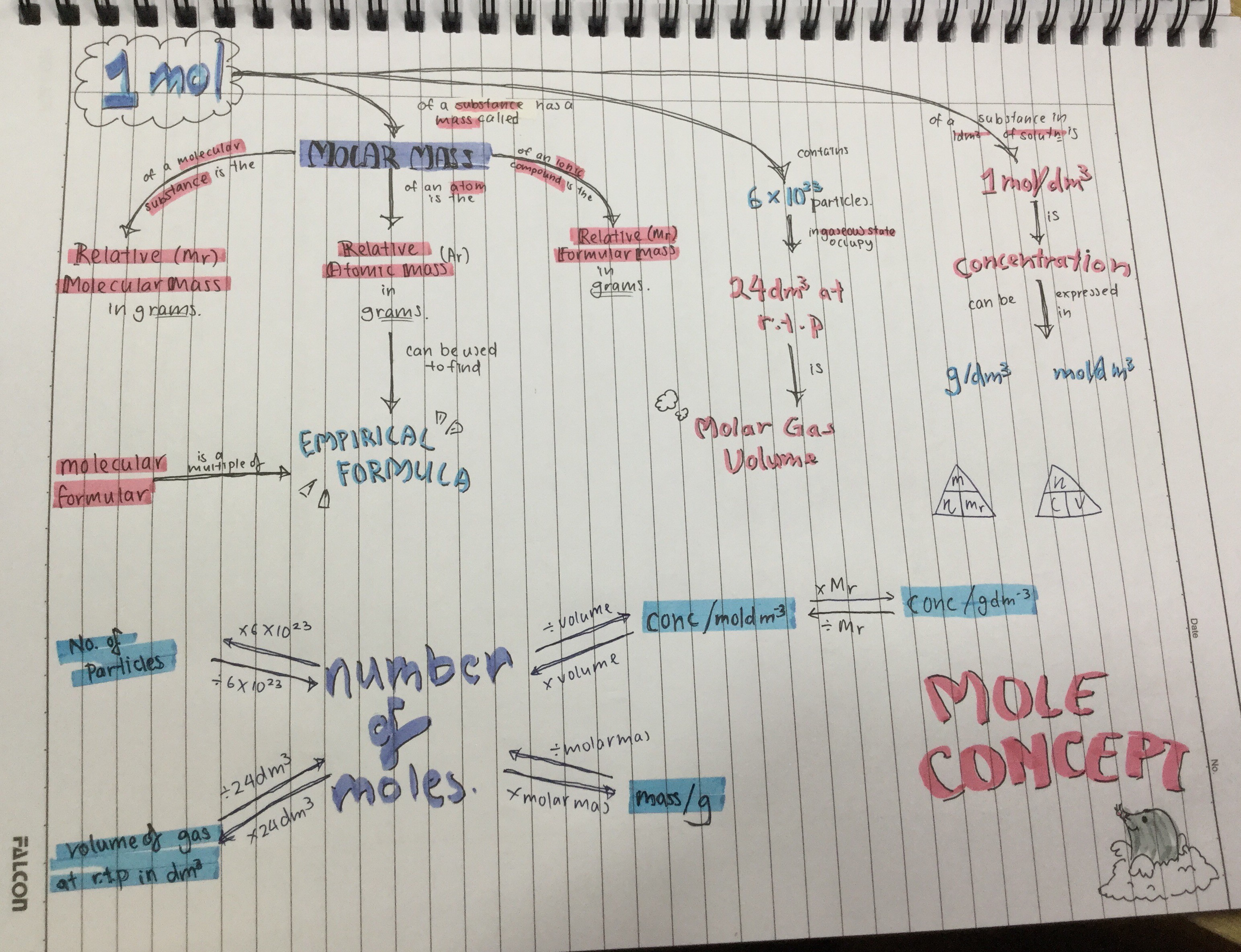
1. 1 mole is 6* 10^23 particles.
2. Relative atomic mass is the mass of 1 mole of atoms.
3. Relative molecular mass is the mass of 1 mole of molecules.
4.From the relative molecular mass, you can find out the empirical formula of a molecule. For example, the relative atomic mass of carbon from the periodic table is 12 and oxygen is 16. If we are given the relative molecular mass of a substance that contains C and O is 44, then we know that there is one atom of carbon and two atoms of oxygen. The empirical formula should then be CO2.
5. The volume of 1 mole of gas is 24 dm3.
6.number of moles /24 is the number of moles of gas in 1 dm3 of gas.
7.concentration= number of moles/volume of gas (expressed in moldm-3) or
= number of moles/mass of as (expressed in molg-1)
After you understand the concepts, you have to try the various questions.
Remember, practice makes perfect.