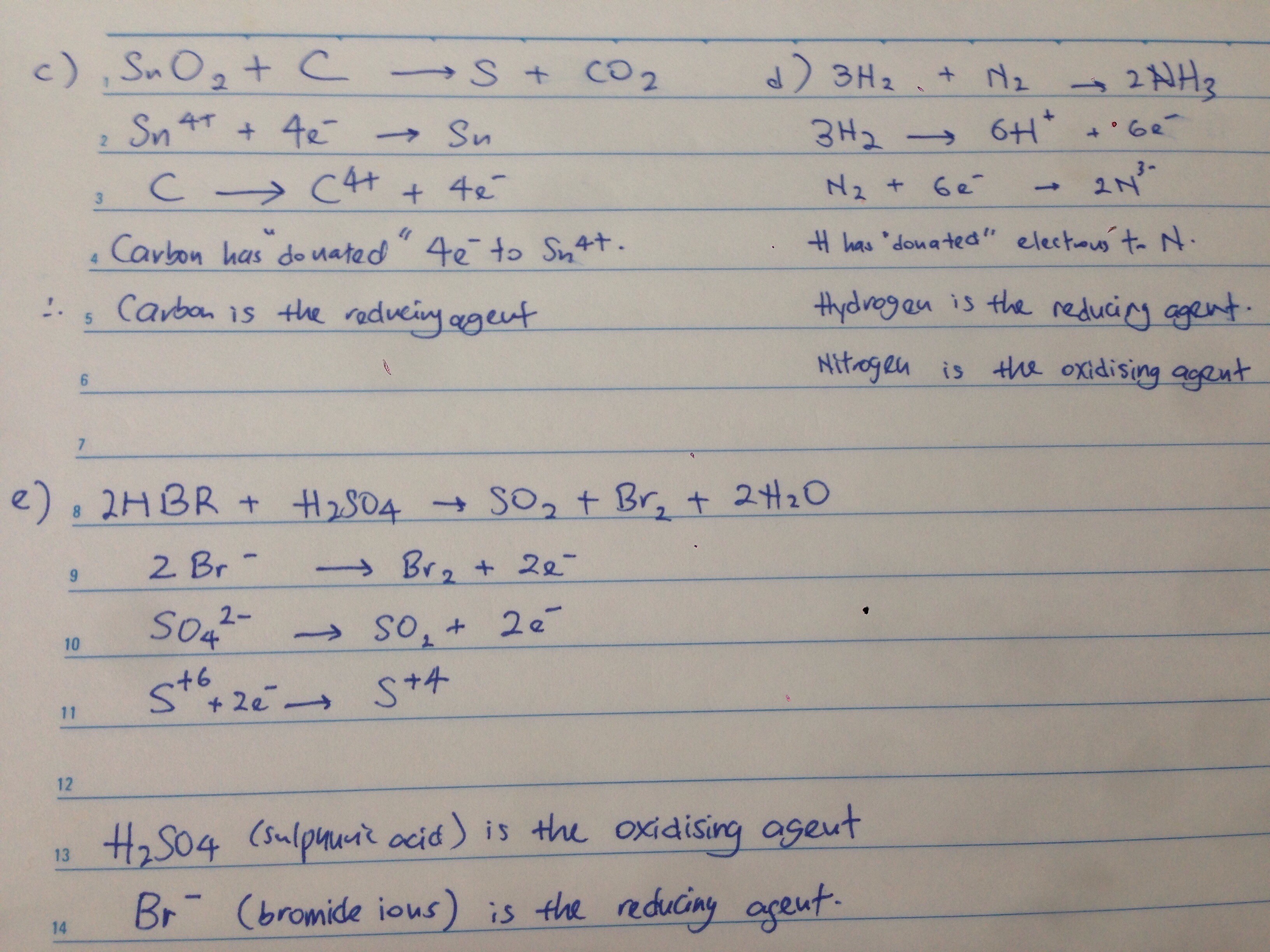Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Focus on the oxidation numbers of each element
Increase in oxidation number = that element has been oxidized
Decrease in oxidation number = that element has been reduced
Quick tip:
In a redox reaction, a reducing agent will usually be oxidized.
And an oxidizing agent will usually be reduced.
Increase in oxidation number = that element has been oxidized
Decrease in oxidation number = that element has been reduced
Quick tip:
In a redox reaction, a reducing agent will usually be oxidized.
And an oxidizing agent will usually be reduced.
Date Posted:
6 years ago