Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 3 | Biology
2 Answers Below
Anyone can contribute an answer, even non-tutors.

(Chemistry) I need some advice on how to study salt preparation. Nothing is getting into my head at all and I can’t remember anything. Would appreciate your help:-(
Ready? GO!
First and foremost,
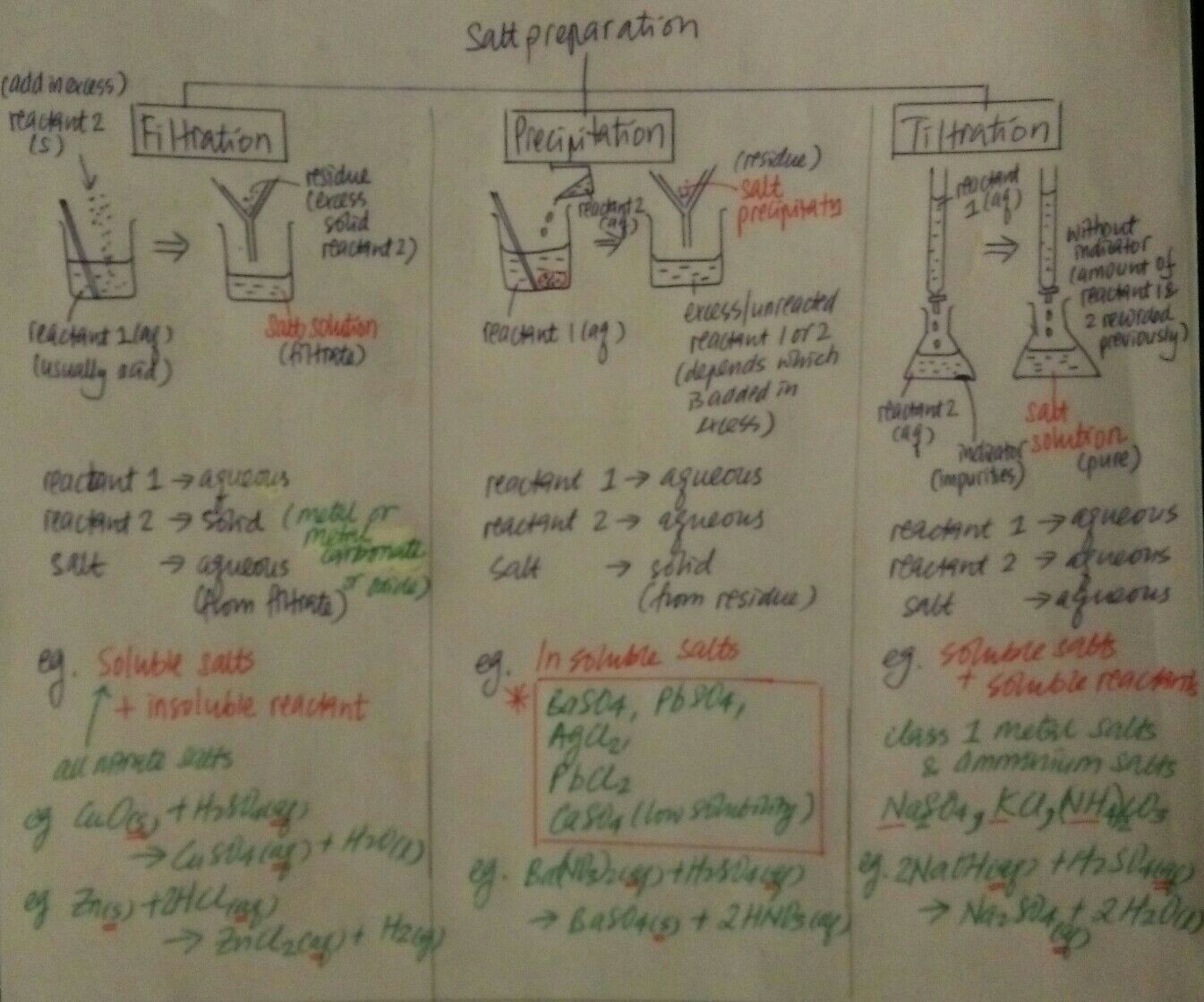
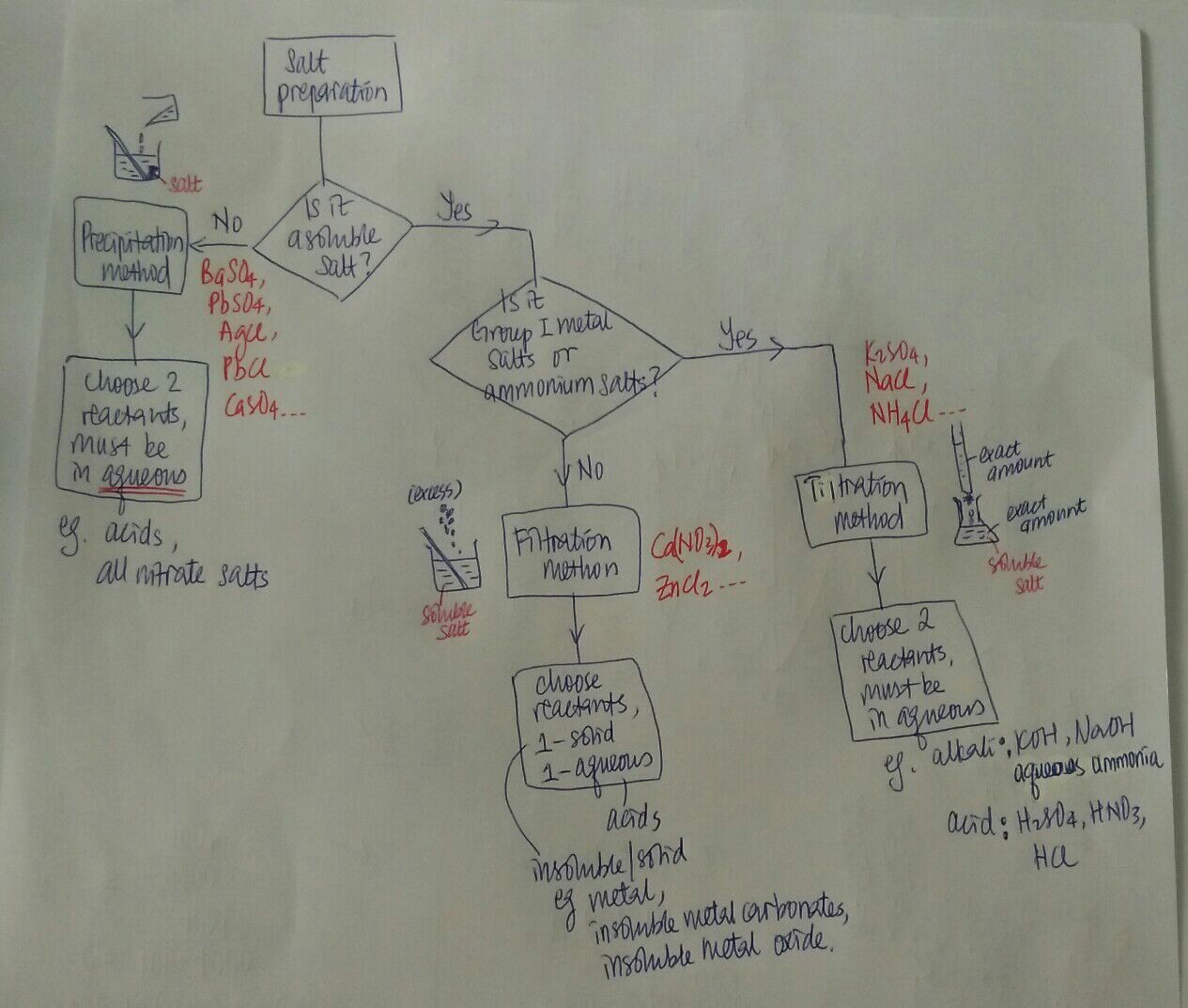
We need to determine if the salt to be prepared is soluble in water.
Pick one salt below that you want to prepare. And tell me if it is soluble in water.
a) Lead Sulfate, PbSO4
b) Zinc Chloride, ZnCl2
c) Sodium Nitrate, NaNO3
a) Lead sulfate: is insoluble...?
For insoluble salt, the method of preparation is the easiest. It is called "Precipitation".
Just think of 2 AQUEOUS reactants that could give you PbSO4.
ie.
Pb?? + ???SO4
Can u identify the 2 reactants?
as long as they are aqueous (soluble in water)
If u have problem with this , do let me know.
Then you get PbSO4 and 2HNO3…?
So since PbSO4 is insoluble just filter out the H2NO3
IM NOT SURE
When we talk about preparation of salt it is not just the chemical equation that produces the salt, but also the consideration of how we can PRACTICALLY obtain a SOLID sample of the salt.
So the equation is:
Pb(NO3)2 (aq) + H2SO4 (aq) --> PbSO4 (s) + 2HNO3 (aq)
Do we need to worry about which one is the limiting reactant? Or any excess reactant?
NO! Because any excess reactant, because it is aqueous, will pass through the filter paper together with the aqueous HNO3 during filtration, and the salt we want is the only substance left behind in the residue. That's why the reactants must be aqueous in this method of precipitation.
One more question..
the salt we want, PbSO4 collected as the residue in the filtration process, will have a film of HNO3 or the excess reactant on it (Just like when we come up after swimming in the sea, there will be a film of sea water on our body) . How do we remove that film of impurities on the PbSO4?
So now, before we move on to the next salt, are you confident of how to prepare insoluble salt, like AgCl2 or CaCO3, etc. .? Do clarify if you have any question on precipitation.
I was wondering if you want to prepare an insoluble salt eg. Calcium sulfate, why can’t you just add calcium into sulfuric acid? In other words just add the metal into acid
let's examine your suggestion of preparing CaSO4(s) using calcium metal with sulfuric acid.. the equation is
Ca (s) + H2SO4 (aq) --> CaSO4 (s) + H2 (g)
So what's wrong with this?
Remember i said earlier that it is not just the equation that produces the salt but also how we PRACTICALLY obtain a solid sample of the salt?
So, what's wrong in the above reaction in terms of pratically obtain a pure solid sample of CaSO4 ? Pause a while to ponder.. :) Share any idea you may have..
Do you agree?
=> meaning that using an aqueous Ca_____ is better than using solid Ca......
You are not confused, you are very clear.
Exactly, the insoluble CaSO4 formed a coating around the remaining Calcium, which prevents furthet reaction! So the yield of CaSO4 is very low, and how are we gonna scratch the CaSO4 off ?! ;) So it is not practical.
Therefore we always use Precipitation to prepare insoluble salt.
That is,
X (aq) + Y (aq) --> insoluble salt + Z (aq)
Do u have further questions before we proceed?
Other than that nope please proceed. This is actually quite effective I didn’t understand anything when I read my notes just now
Tempt have to be above 842 degree celcius as that is the melting point of calcium.
Reaction speed will be very fast with the high tempt, solid CaSO4 still can form a coating around some molten calcium, unless it is above 1400 plus celcius, which is the melting point of CaSO4..
Not forgetting at such high tempt, Calcium will form insoluble Calcium Oxide (m.p. 2572 degree celcius) with steam, if present in excess with respect to the acid will make it hard to seperate from CaSO4..
Hmm.. i don't think it is practical. :)
Again, the first question to ask is whether it is soluble in water.
Answer is Yes, ZnCl2 is soluble in water.
Now, to prepare a salt that is soluble in water, we make use of The REACTIONS OF ACIDs.
Acid reactions mean one of the 3 reactions:
1) Acid + Base
2) Acid + Metal
3) Acid + Carbonates
So you see, preparation of salt is quite easy, just remember that
- For INsoluble salt, use ANY X(aq) + Y(aq) reactions (precipitation)
- For Soluble salt, use one of the 3 acid reactions.
If you remember this, the rest will fall into place.
To continue, can you write the equation (with state symbols) of any acid reaction that could produce ZnCl2 ?
Zn(s) +2HCl(aq) -> ZnCl2(aq) + H2(g)
We can also use:
ZnO (s) + 2HCl (aq) --> ZnCl2 (aq) + H2O (l)
or
ZnCO3 (s) + 2HCl (aq) --> ZnCl2 (aq) + H2O (l) + CO2 (g)
As long as it is one of the 3 reactions of acid.
Ok, now back to the reaction you use:
Zn (s) + 2HCl (aq) --> ZnCl2 (aq) + H2 (g)
So how do we practically obtain a pure sample of solid ZnCl2 from this reaction?
One of the reactant could be limiting, the other will be in excess.
If HCl (aq) is in excess then when all the Zn (s) has reacted away and the reaction stops, there will be the salt ZnCl2(aq) produced and the excess HCl(aq) present in the beaker. Both are aqueous and we have a problem seperating the two.. This is the main issue!
So, any idea how to isolate the salt that is produced?
But if HCl is in excess I don’t know how to separate them since both are aqueous :-(
So we must use excess of the solid reactant, to avoid the situation where we are left with a mixture of the aqueous reactant and aqueous product that is hard to seperate.
Let's call this method the "Excess Solid Reactant" method.
You are doing well so far. Do you have further questions to clarify before we proceed to the last salt?
1) Is it possible to separate HCl(aq) and ZnCl(aq)?
2) Also, does insoluble= non-reactive?
I’m quite confused because one of the ways to prepare a salt is acid+ carbonate, but aren’t all carbonates insoluble? Just like the reaction will not be complete if you just add Ca(s)+H2SO4(aq) because CaSO4 is insoluble and it prevents further reaction. So if you add acid and carbonate will the carbonate react in the first place?
Ok the next salt is sodium nitrate, it’s soluble, I think you can obtain it by titration?
So => NaOH(aq) + HNO3(aq) -> NaNO3(aq) + H2O(l)
Edit: I realised there are some soluble carbonates...
1) is it possible to seperate HCl(aq) and ZnCl2(aq)?
Not sure but I guess if we heat the mixture solution to dryness, HCl could escape as a gas.. but then HCl gas will become very corrosive when we breadth it in or when it comes in contact with moisture in our skin/body. So even if this is possible it is not recommended.
2) Does insoluble = unreactive?
Answer is No.
For example:
- Calcium Carbonate is insoluble but it will react with acids, as acids will react with carbonates.
- ZnO is insoluble but it will react with acid, as acid will react with a base.
Do not confuse by the case of
Ca(s) + H2SO4(aq) --> CaSO4 (s) + H2(g)
Here the insoluble product CaSO4 forms a solid coating around the Ca metal, because CaSO4(s) does not react with acid (is it one of the acid reaction? No) .
Right, you correctly identified that it is soluble in water, and hence use an acid reaction to produce it. And you have chosen the reaction of acid + base:
NaOH(aq) + HNO3(aq) --> NaNO3(aq) + H2O(l)
And you said Titration . Do u know why here you need to do titration first?
Titration to neutralise the base?
Why can't we have excess HNO3(aq), or for that matter, excess NaOH(aq) ?
I’m not sure what we’re trying to find
We can’t have excess because we are using an indicator so we know that the end point is reached when the indicator changed it’s colour. I’m not very sure but I think if we put excess in titration it’s quite meaningless.
But the question is, why do we need to do titration at all? Why not we don't do titration and just react any amount of NaOH(aq) and HNO3(aq) together, hence allowing one of the reactant to be in excess? Afterall the salt we want NaNO3 (aq) will still be formed and that is what we want right?
Recap: in preparing the previous salt ZnCl2, why wee need to add the solid Zn metal in excess rather than add HCl(aq) in excess?
See 2 Answers
Pls see the second photo, you need to ask these questions in sequence, in order to choose the correct method...