Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
junior college 2 | H2 Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

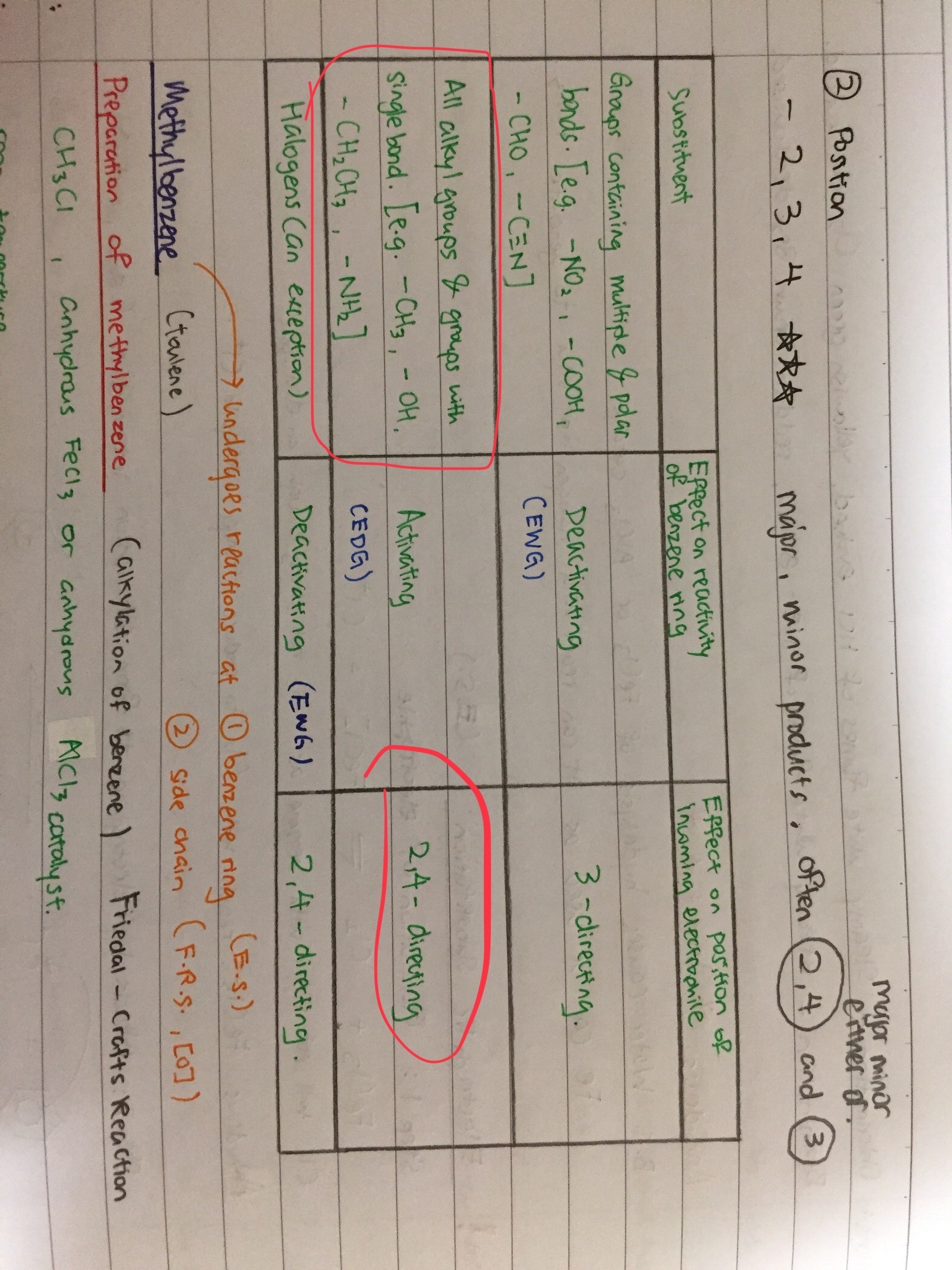
Q29 pls
It is more likely that other (unstable) part will form reaction first. (ie CH3 and NH2)
For D, the Cu2+ ionic solution (note that it is ions so they are charged and ready to attach to anywhere unstable)
See 1 Answer