Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.
Information will be in comments, thankyou!!
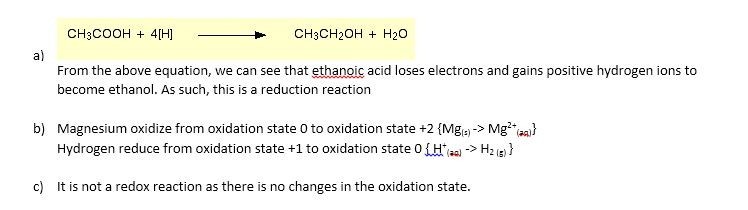
CH3OOH(aq) - LiAlH4-> CH3CH2OH---Reaction 1
2CH3COOH (aq) +Mg (s) - > (CH3COO) 2 Mg (aq) +H2 (g) - - - - Reaction 2
CH3COOH (aq) +NaOH (aq) - > CH3COONa (aq) +H2O (l) - - - Reaction 3
See 1 Answer
Hint for part (i), the products are already stated in the question. You just need to balance the equation.
Hint for part (ii), there is no change in the oxidation state of carbon