Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

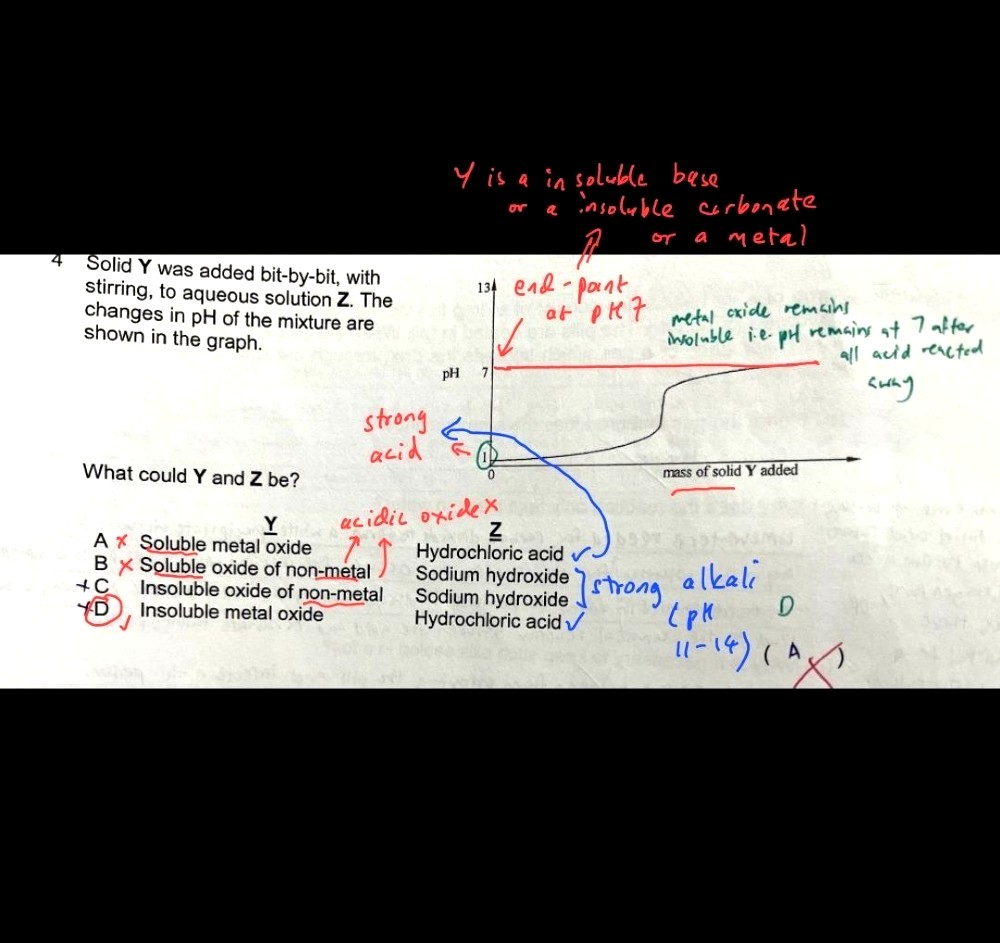
can someone explain to me why D is the answer? i really dont understand
This means that the pH of the resulting solution would be around 7 after complete reaction.
If we use alkali instead, an excess of the alkali will result in a pH level above 7.
See 1 Answer