Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | E Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

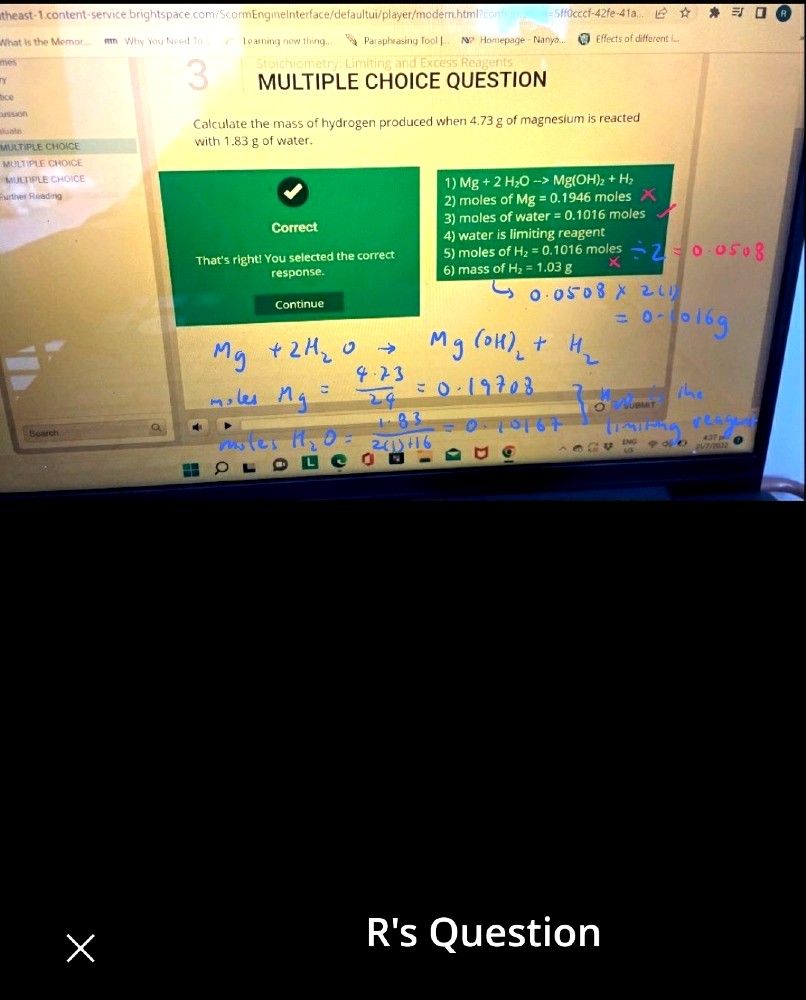
Hi can someone please tell me if I’m tripping or is the mass of H2 =1.03g here wrong
Cuz isn’t the Mr of H2 here (4 times 1.01)
Since there are 4 H produced.
So isn’t it 0.1016moles times (4*1.01) = 0.41046g
So why is the answer 1.03g?
Pls help me make sense thank u!
See 1 Answer