Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
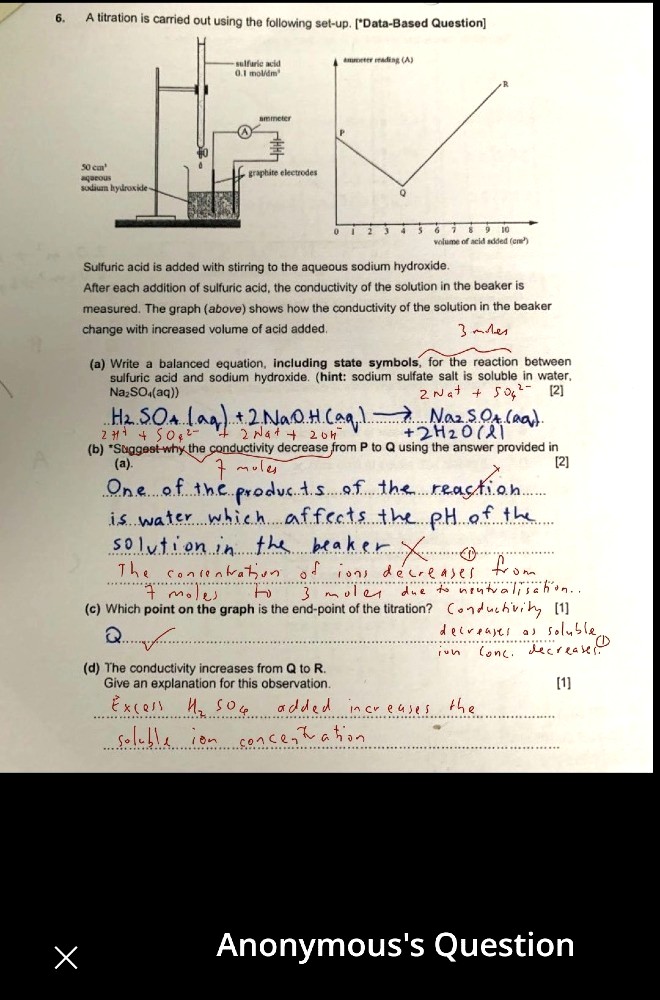
hi, why do u need to write the ionic equation of the reaction first then compare the number of moles? why cannot compare directly?
You can compare directly, if you know how many moles of soluble ions are present for the reactants and for the products. If not sure, best to write out the ions for better understanding. Note that what I wrote out is not the simplified and final ionic equation.
oh so either way you still need to compare the number of moles of ions on both sides, just that writing the ionic eqn would make it easier to see?
More or less. Basically, you have to understand that electrical conductivity of solutions varies proportionately to the concentration of ions present. ( or in this case, number of ions, since concentration was not provided).
ohh I see, thank you
Np