Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
2 Answers Below
Anyone can contribute an answer, even non-tutors.

Why is the answer b?
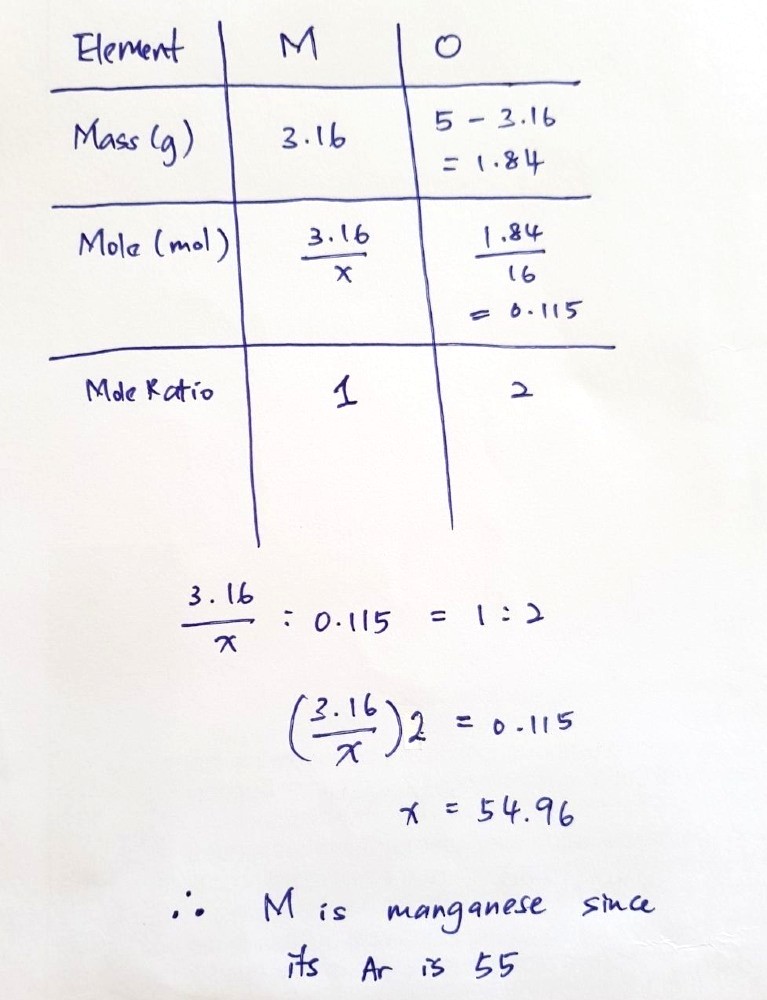
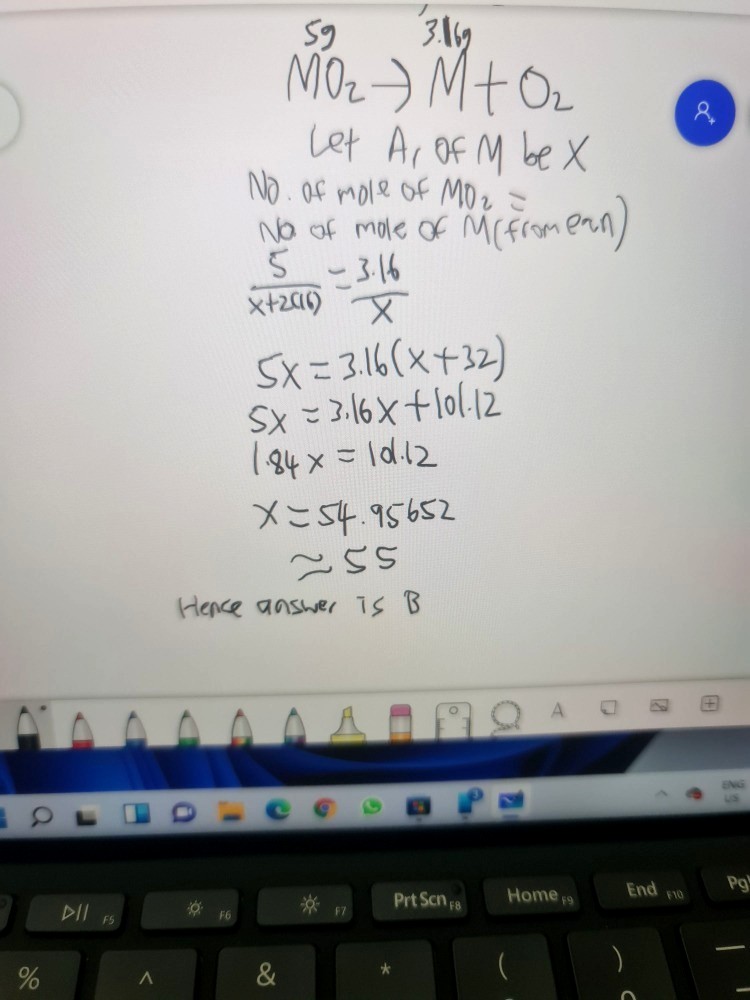
Number of moles of oxygen reached
= 1.84 / 16 (because relative atomic mass of oxygen is roughly 16)
= 0.115
Because the ratio M : O in the solid is 1 : 2, we expect 0.0575 moles of metal to react with it.
This means that the relative atomic mass of M
= 3.16 / 0.0575
= 54.95…
From here, we see that manganese, with a relative atomic mass of 55, is probably the best option here.
See 2 Answers