Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 2 Answers
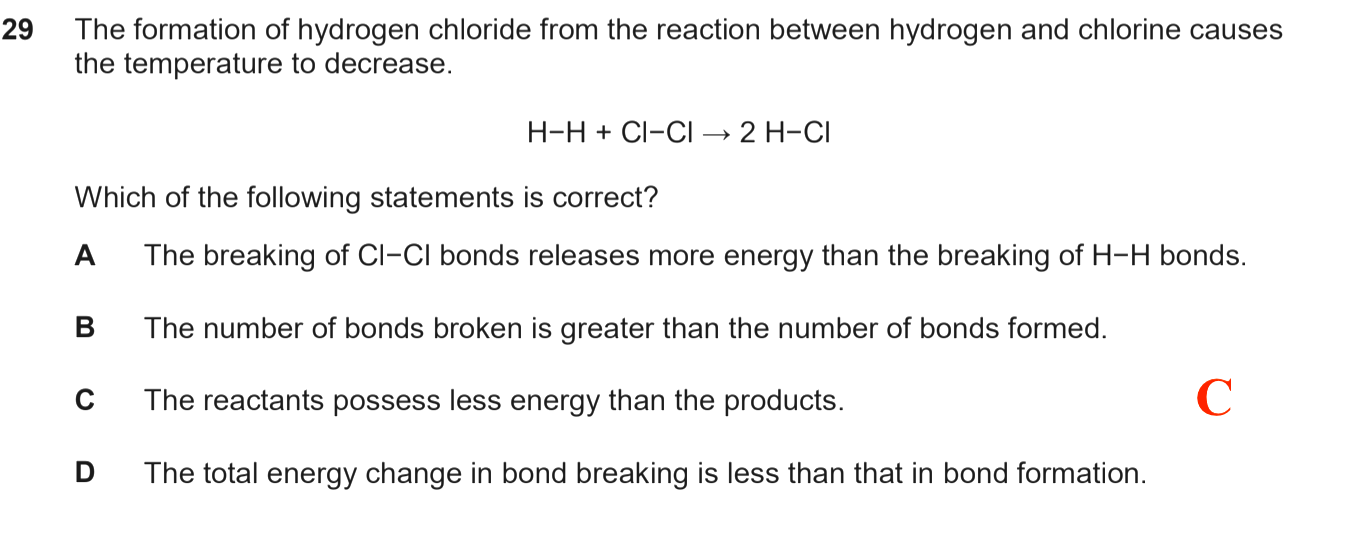
Temperature decrease means the reaction takes in (heat) energy overall ( this is called an endothermic reaction)
So, the total energy needed to break the H-H and Cl-Cl bonds is higher than the energy released when the two H-Cl bonds are formed.
A is obviously wrong as bond breaking requires energy, not releases energy.
B is wrong because two bonds are broken, and two bonds are formed (i.e same number)
D is wrong (see above explanation)
C is correct because the endothermic reaction means there is a net gain in energy. The products thus have more energy than reactants.
So, the total energy needed to break the H-H and Cl-Cl bonds is higher than the energy released when the two H-Cl bonds are formed.
A is obviously wrong as bond breaking requires energy, not releases energy.
B is wrong because two bonds are broken, and two bonds are formed (i.e same number)
D is wrong (see above explanation)
C is correct because the endothermic reaction means there is a net gain in energy. The products thus have more energy than reactants.