Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.

How to find (b), (c) & (e)?
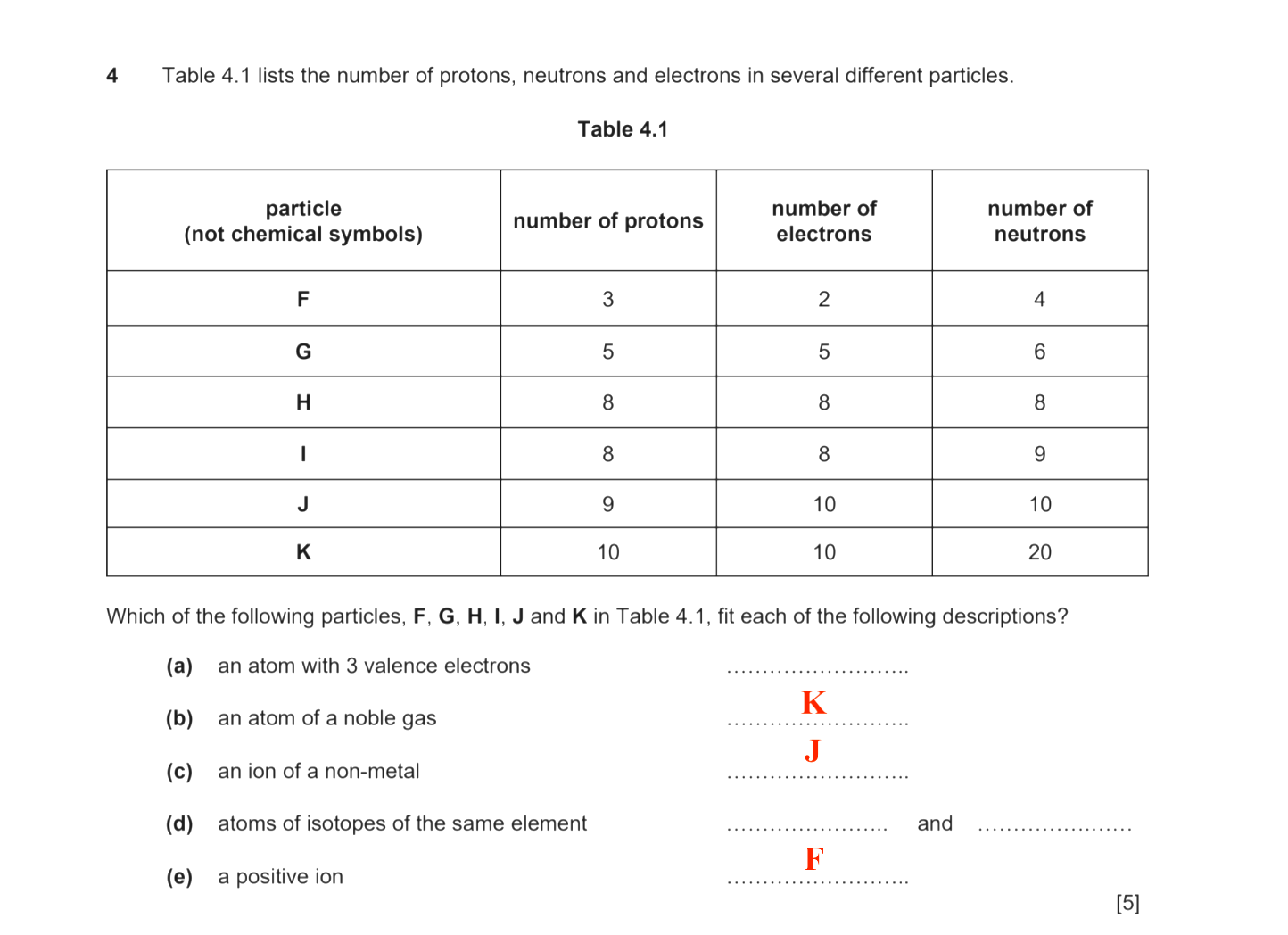
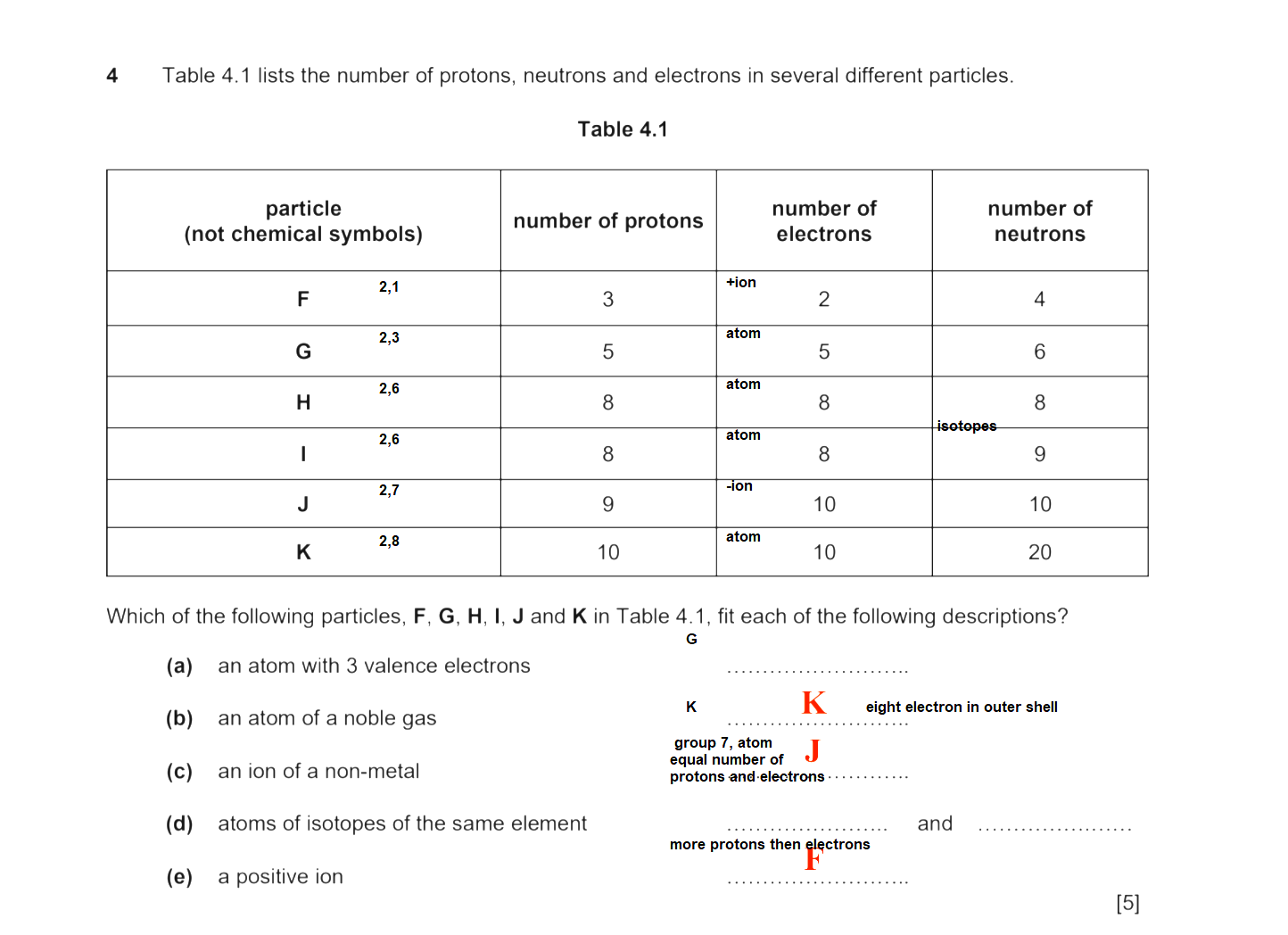
Particle F
3 protons → Lithium (Li)
2 electrons → 1 less than proton number → singly positively charged ion (cation)
4 neutrons → the usual isotope since mass/nucleon number - proton/atomic number = 7 - 3 = 4
So, Particle F is a lithium cation (Li⁺)
Fits the answer for e)
5 protons → Boron (B)
5 electrons → same as proton number → neutral atom
6 neutrons → the usual isotope since mass/nucleon number - proton/atomic number = 11 - 5 = 6
So, particle G is a boron atom (B)
It has 2 electrons in the first shell, and 3 in the 2nd shell (which is its outermost shell)
So it fits the answer for a) since there are 3 outermost/valence electrons.
8 protons → Oxygen (O)
8 electrons → same as proton number → neutral atom
Particle H
8 neutrons → the usual isotope since
mass/nucleon number = 8 + 8 = 16
Particle I
9 neutrons → not the usual isotope since mass/nucleon number = 8 + 9 = 17
So , Particle H is a oxygen-16 atom (¹⁶O) and Particle I is a oxygen-17 atom (¹⁷O)
Fits the answer for d) since they are atoms of isotopes of the same element.
9 protons → Fluorine (F)
10 electrons → one more than proton number → singly charged negative ion (anion)
9 neutrons → the usual isotope since mass number = 10 + 9 = 19
Particle J is a fluorine anion (F⁻)
Fits the answer for c) since fluorine is a non-metal
10 protons → Neon (Ne)
10 electrons → same as proton number → neutral atom
20 neutrons → not the usual isotope since mass number = 10 + 20 = 30
(The mass number in the perodic table is 30)
So Particle K is a neon-30 atom (³⁰Ne)
Neon is in group 18 (or also known as Group VIII or Group 0), which is the noble gas group
It has 8 electrons in the outermost shell (8 valence electrons)
Fits the answer for b)
See 1 Answer
Group 7 is for another group (transition metals Manganese, Technetium Rhenium, etc)
The equivalent group for Particle J is Group 17