Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | Chemistry
One Answer Below
Anyone can contribute an answer, even non-tutors.

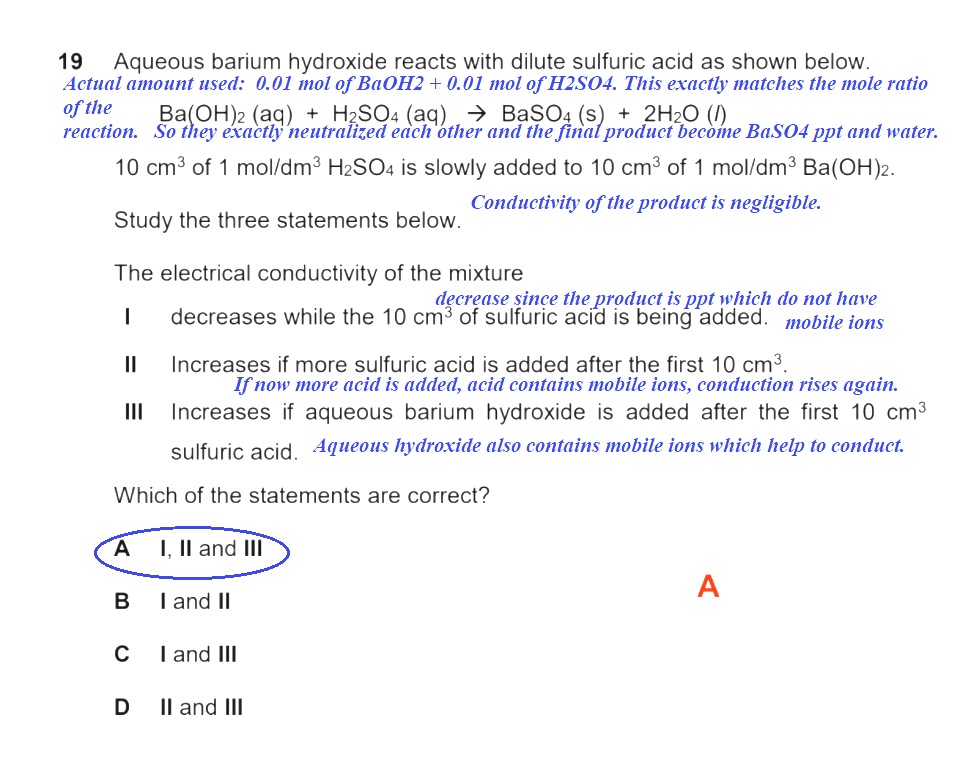
How come A is the answer?
==> barium sulfate is an insoluble salt which does not conduct electricity
==> as the H2SO4 is being added, barium sulfate is being formed; this reduces the otherwise available ions available in the solution (barium hydroxide is soluble to a certain degree)
==> with less ions over time, the solubility is reduced
If you actually do the mole ratios, you will notice that the H2SO4 exactly neutral uses the Ba(OH)2. This is when the maximum quantity of insoluble BaSO4 exists and when the minimal electrical conductivity is in place.
The moment you add H2SO4 or Ba(OH)2 from that point on, there is no further reaction, so the ions get resupplied once again and electrical conductivity increases once again.
See 1 Answer