Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

Question
secondary 4 | A Maths
One Answer Below
Anyone can contribute an answer, even non-tutors.

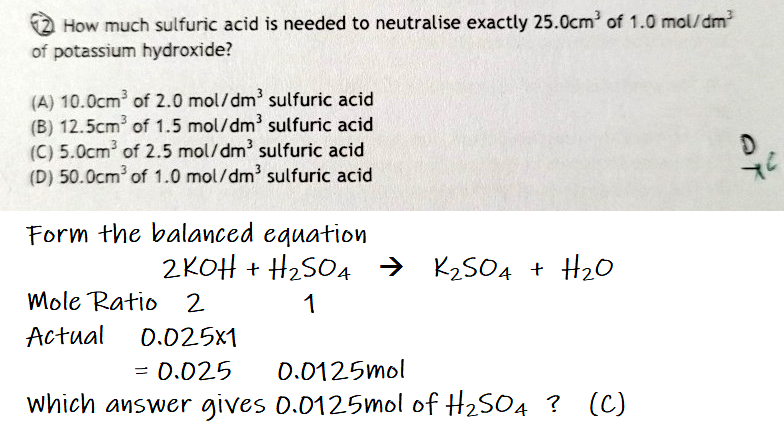
need help with this qn, pls explain too
LockB, you need to write the balanced equation to realise that the mole ratio of potassium hydroxide to sulfuric acid is 2 : 1 (we need this for the computations).
why is this wrong tho
1. I know that there are two hydrogen ions for every H2SO4.
2. I also know that every hydrogen ion reacts with exactly one hydroxide ion to allow the neutralisation to proceed (meaning to say that two hydrogen ions require two hydroxide ions for neutralisation).
3. However, the two hydrogen ions come in the form of one molecule of H2SO4 while the two hydroxide ions come in the form of two separate molecules of KOH.
4. This means that one molecule of H2SO4 reacts with two molecules of KOH.
5. This also means that one mole of H2SO4 reacts with two moles of KOH.
6. It is worth noting, in your case, that the hydrogen ion to hydroxide ion ratio is 1 : 1 (leading to why you felt that the mole ratio should be 1 : 1).
7. However, in actual reality, the reactants come in the form of H2SO4 molecules and KOH molecules rather than random hydrogen ions and hydroxide ions, so the actual mole ratio should be based on actual molecules rather than the ions themselves,.
See 1 Answer