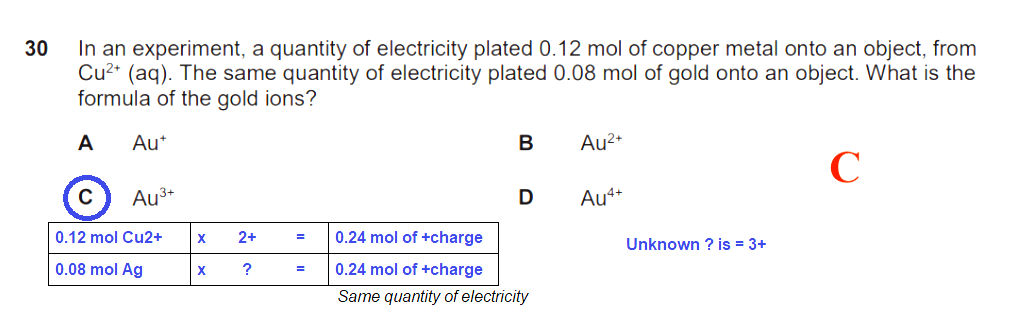Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 2 Answers
every Cu²+ ion needs 2 electrons to become an atom of Cu.
Since 0.08/0.12 = 2/3, the ratio of Au atoms formed to Cu atoms formed with the SAME number of electrons supplied is 2 : 3.
This means the ratio of number of electrons taken in per Au atom to that per Cu atom is 3 : 2.
So Au³+
Since 0.08/0.12 = 2/3, the ratio of Au atoms formed to Cu atoms formed with the SAME number of electrons supplied is 2 : 3.
This means the ratio of number of electrons taken in per Au atom to that per Cu atom is 3 : 2.
So Au³+