Ask Singapore Homework?
Upload a photo of a Singapore homework and someone will email you the solution for free.

See 1 Answer
done
{{ upvoteCount }} Upvotes
clear
{{ downvoteCount * -1 }} Downvotes
Correct?
Date Posted:
2 years ago
Actually...
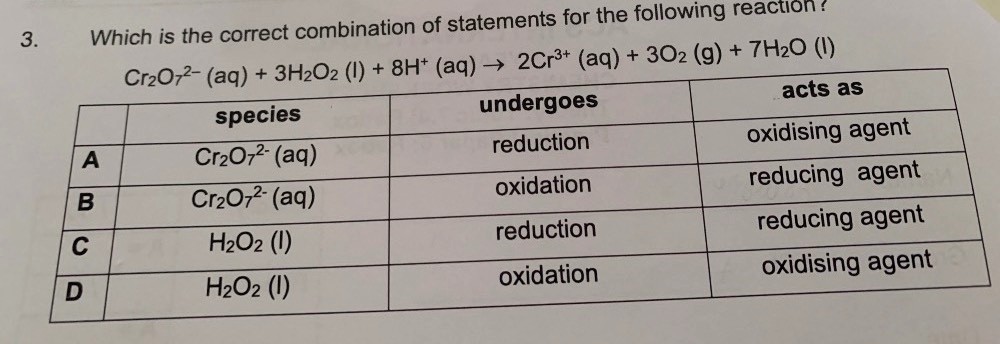
The Cr in “Cr2O7 2-“ has an oxidation state of +6 each; we count the oxidation state by the respective element.
So in fact, the Cr sees a reduction from +6 to +3 (typical of chromium ions). So, the Cr2O7 2- ions is actually reduced.
The Cr in “Cr2O7 2-“ has an oxidation state of +6 each; we count the oxidation state by the respective element.
So in fact, the Cr sees a reduction from +6 to +3 (typical of chromium ions). So, the Cr2O7 2- ions is actually reduced.
The H2O2, ironically enough, faces possible oxidation and reduction. The oxidation state of O here is -1 (in the rare exception).
The oxidation is possible (because the oxidation state of O in O2 is 0).
The reduction is possible (because the oxidation state of O in H2O is -2).
But sadly, the Cr2O7 2- ions has been reduced to Cr 3+ ions (and is therefore an oxidising agent), so H2O2 has to take the role of the reducing agent.
The oxidation is possible (because the oxidation state of O in O2 is 0).
The reduction is possible (because the oxidation state of O in H2O is -2).
But sadly, the Cr2O7 2- ions has been reduced to Cr 3+ ions (and is therefore an oxidising agent), so H2O2 has to take the role of the reducing agent.